One of the 10 Most Valuable New Drugs under Development - A Comprehensive View of Potential Heavyweight Drug Patents
Evaluate Pharma recently published an article listing the "most valuable and promising drugs" currently under development, including 10 drugs such as MSD's ACVR2A-Fc fusion protein sortercept,Novartis' CFB inhibitor iptacopan, and Madrid's heavyweight new drug in the NASH field, resmetirom.
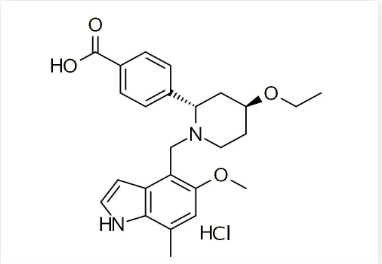
表1. Evaluate预测最有价值的10款在研新药According to the author's organization, there are a total of four chemical drugs, namely Iptacopan, Aficamten, Resmetrirom, and Karxt (a compound formulation of Zanomelin Tartrate and Tristanium Chloride). The following text summarizes the core patent information of these four heavyweight drugs for industry reference.Iptacopan is the first oral complement system regulatory factor B targeted inhibitor developed by Novartis AG in Switzerland. It amplifies circulation by inhibiting the B factor pathway and is used to treat complement driven kidney disease (CARD).
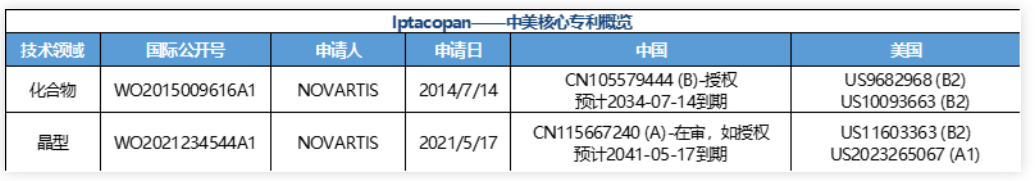
Latest progress: NDA (2023-06-14, China applies for listing). In June 2023, CDE officially accepted the marketing approval application for iptacopan (LNP023) capsules and included them in the priority evaluation process. Its indication is adult paroxysmal nocturnal hemoglobinuria (PNH). This breakthrough drug will bring new treatment options for PNH patients, helping them regain their health and hope.Overview of core patents: Compound (general formula): WO2015009616 (A1), applied for on July 14, 2014, has entered China and the United States. Among them, China: Patent CN105579444 (B) has been authorized and is expected to expire on July 14, 2034; Two authorized patents in the United States, US9682968 (B2) and US10093663 (B2).Crystal form (crystal hydrate of hydrochloride): WO20212345442021-05-17 application, has entered China and the United States. Among them, China CN115667240 (A), 2021-05-17 application, status is under review; United States: US11603363 (B2) authorized.
表2. Iptacopan中美核心专利;A代表发明公开,B代表发明授权
First examination opinion: Claims 18, 20-21 do not comply with Article 26 (4) of the Patent Law.
The expression "especially" appears in claims 18 and 20, which limits the scope of protection of the claims to different levels, making their scope of protection unclear and not in compliance with Article 26 (4) of the Patent Law.Second examination opinion: Claim 15 does not comply with the provisions of Article 26 (4) of the Patent Law.
The first compound in claim 15 is named "w1- (5,7-dimethyl-1H -- yl) methyl) -2-yl-4-ol", where "w" makes the scope of protection of the claim unclear and does not comply with Article 26 (4) of the Patent Law.It can be seen that during the examination process of this patent, the examiner did not raise any questions of novelty or creativity, indicating that the patent has a certain degree of stability.Aficamten is a small molecule drug developed by Cytokines Inc. It is a cardiac myosin complex inhibitor and currently has its highest development stage in clinical phase III for the treatment of hypertrophic cardiomyopathy.
Overview of core patents: compound (general formula), WO2019144041A12019-01-18 application; It has entered China and the United States. Among them, China CN111757875 (A), status is under review; The United States has authorized two patents, US10836755 (B2) and US11472796 (B2).Crystal form (polycrystalline form): WO2021011809A12020-07-16 application, has entered China and the United States. Among them, China: CN114667283 (A), status is under review; US: US2022274969 (A1), status is under review.